half life formula for first order reaction
You will have to substitute A in the differential equation and solve it in order to obtain the rate equation in this case. T ½ A o 2k For a first order reaction A products rate kA.
The First Order Half-Life calculator computes the first order half-life based on the temperature dependent rate constant.
. What is the half-life of a first-order reaction with a rate constant of 870104 s1. The half-life of a zero-order reaction can be calculated by rearranging the equation. D A d t k A If the volume is V t then A n V t.
The half-life of a reaction is referred to as t 12 unit - seconds The initial reactant concentration is referred to as R. Using the half-life equation derived from the concentration-time equation as shown in example 1 we can solve for the initial concentration of reactant. Converting a half life to a rate constant.
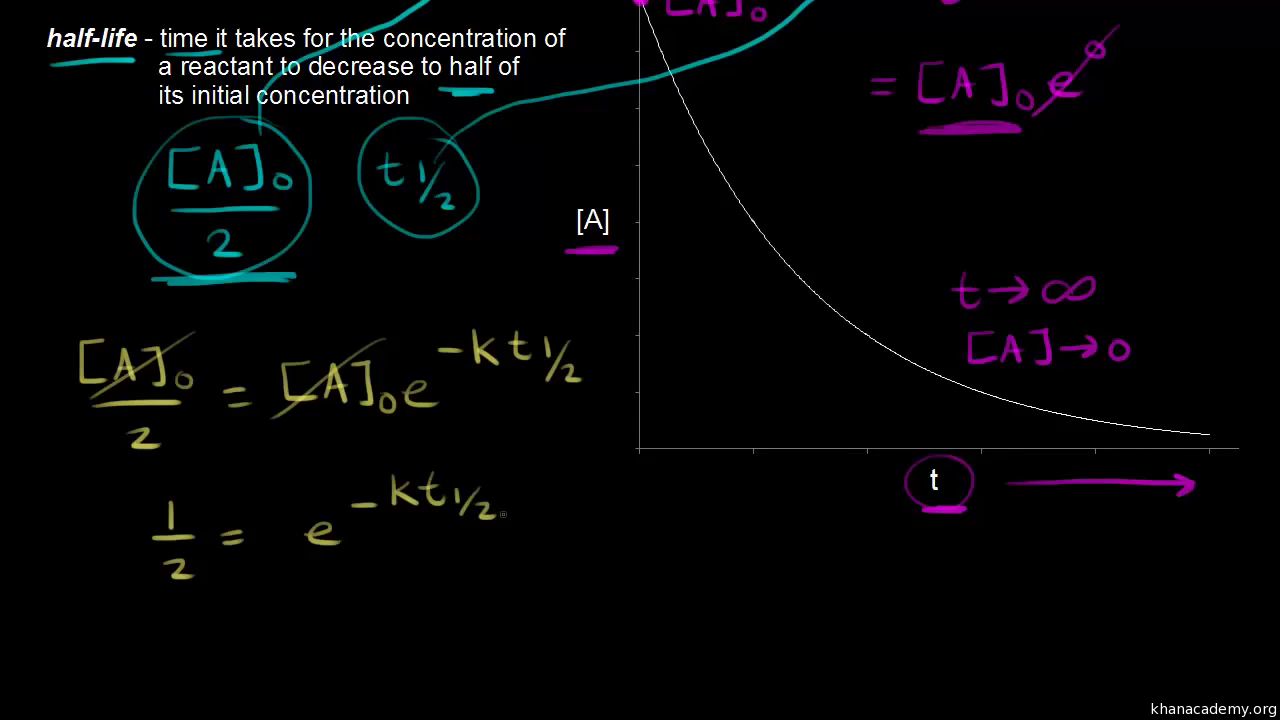
Ln N o N t -λt i ii where N t is the concentration of remaining. The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02. T 12 R 0 2k.
Notice that the half life does not depend on the reactant concentration. What is the rate constant of a first-order reaction that takes 533 seconds for the reactant. For the first order reaction you can plug the definition of the half life into the concentration-time reaction to obtain a neat relationship.
The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. Graphical relations and half lives. The rate law for this first order rate equation is where A is the concentration of radium-223 at time t and k is the rate constant 00606 day -1.
The half-life of a first-order reaction does not depend upon the concentration of the reactant. K 2303t log R 0 R At t t 12 R R 02 according to the reaction half-life definition. The half-life of a second-order reaction is given by the formula 1kR 0.
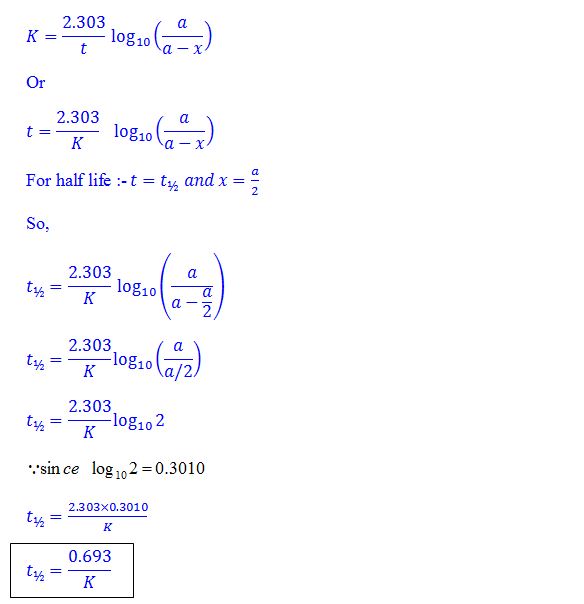
The following equation is. The integrated rate law of first-order reaction is given as. Deriving half-life equation of a first-order reaction starting from the integrated rate lawThe best books that can be referred to for JEE Mains and Advance.
Find more Chemistry widgets in WolframAlpha. Half Life of First Order Reactions First-Order Reactions We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows. Numericals on zero order reactions.
Integrated Rate Law Equation for Zero Order Reaction. T120693k where t12 is the half-life in seconds s and k is the rate constant in inverse seconds s1. The general equation of first-order kinetics.
T ½ 1 k A o Top. It is a constant and related to the rate constant for the reaction. Substituting the values in the expression for the rate constant of half-life first-order reaction the.
For first order reactions assuming the reaction A B C. T_12 frac1A_0k. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2.
Derivation of Half-Life Formula for First Order Reaction. Equations for Half Lives. For a zero order reaction A products rate k.
The half life of a first order reaction is 100 seconds at 280 K. The half-life of a first-order reaction is given as t 12 0693k. Determining a half life.
Ln 12A0A0 -kt12. You want to calculate the time it takes for the concentration to fall from A 0 to ½ A 0. The following is the formula for a first-order rate constant expressed mathematically.
This is because radioactive decays always follow first-order kinetics and they can be calculated with an integrated half-life equation. FraclnA_0A_tkt tfraclnA_0A_ttimesfrac1k. Half-life equation for first-order reactions.
Ln 2 0693 kt12. Get the free Half Life Calculator first order reaction widget for your website blog Wordpress Blogger or iGoogle. Plot the graph between Concentration Rate and Time for Zero Order Reactions.
T 12 0693k.

Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube

First Order Reactions Study Material For Iit Jee Askiitians

First Order Reaction Definition Example Half Life Period Chemist Notes
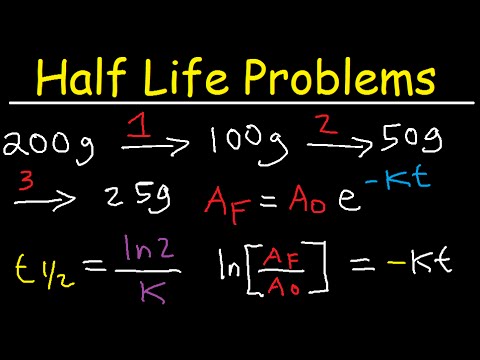
Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube
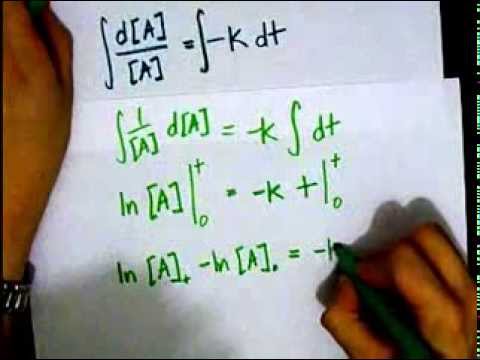
Integrated Rate Law First Order Reaction Youtube
Zero Order Reactions Video Kinetics Khan Academy

Half Life Of A First Order Reaction Video Khan Academy

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
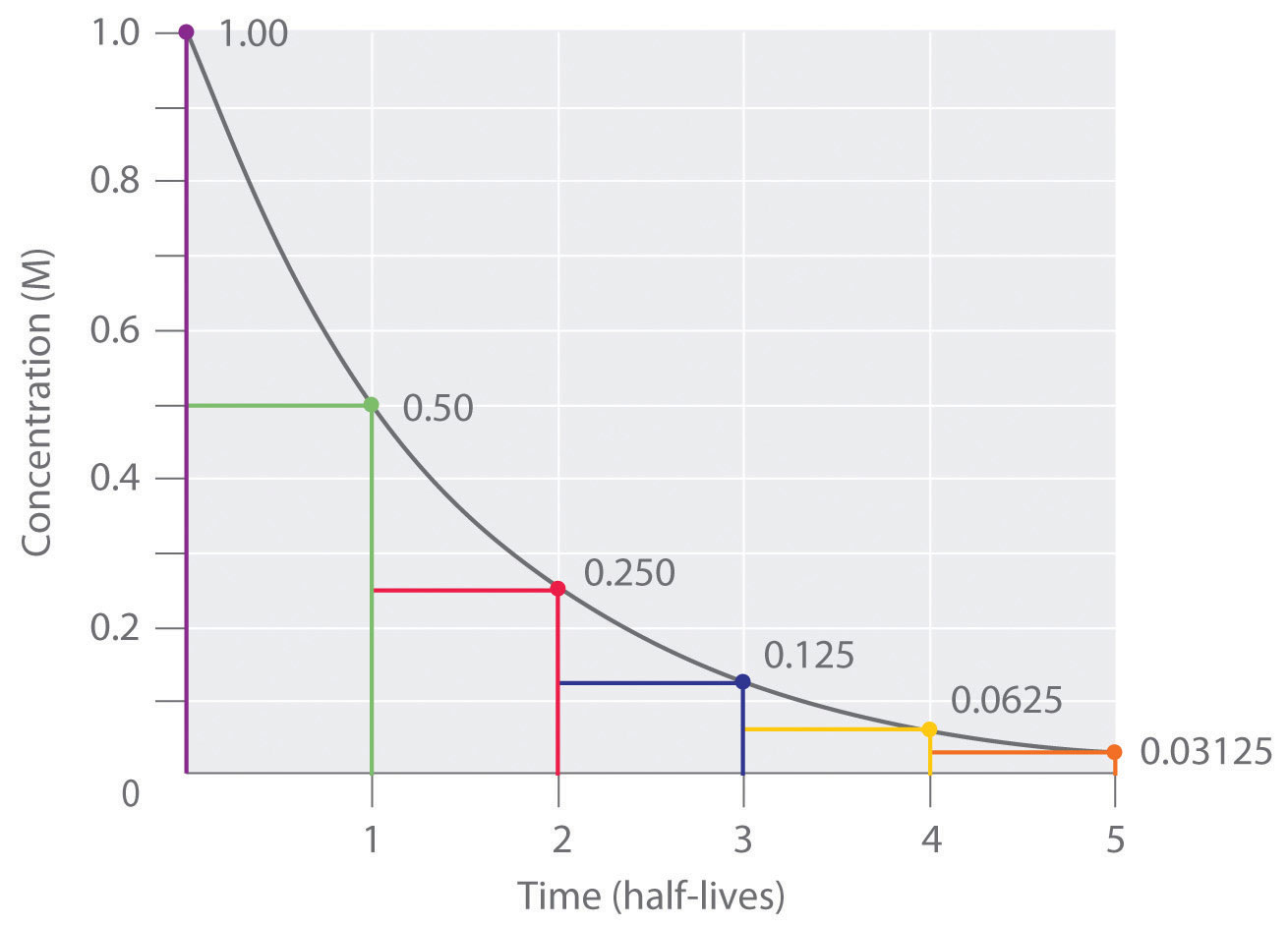
4 5 First Order Reaction Half Life Chemistry Libretexts
Half Life Of A First Order Reaction Video Khan Academy

First Order Reaction Chemical Kinetics First Order Reaction Youtube

First Order Reaction Overview Equation What Is Rate Law Equation Video Lesson Transcript Study Com
What Is Meant By Half Life Prove That Half Life Period Of First Order Reaction Does Not Depend On Its Initial Concentration Answer Mp Board Class 12 Chemistry Question Answer Collection

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

First Order Reaction Definition Examples And Equations

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1